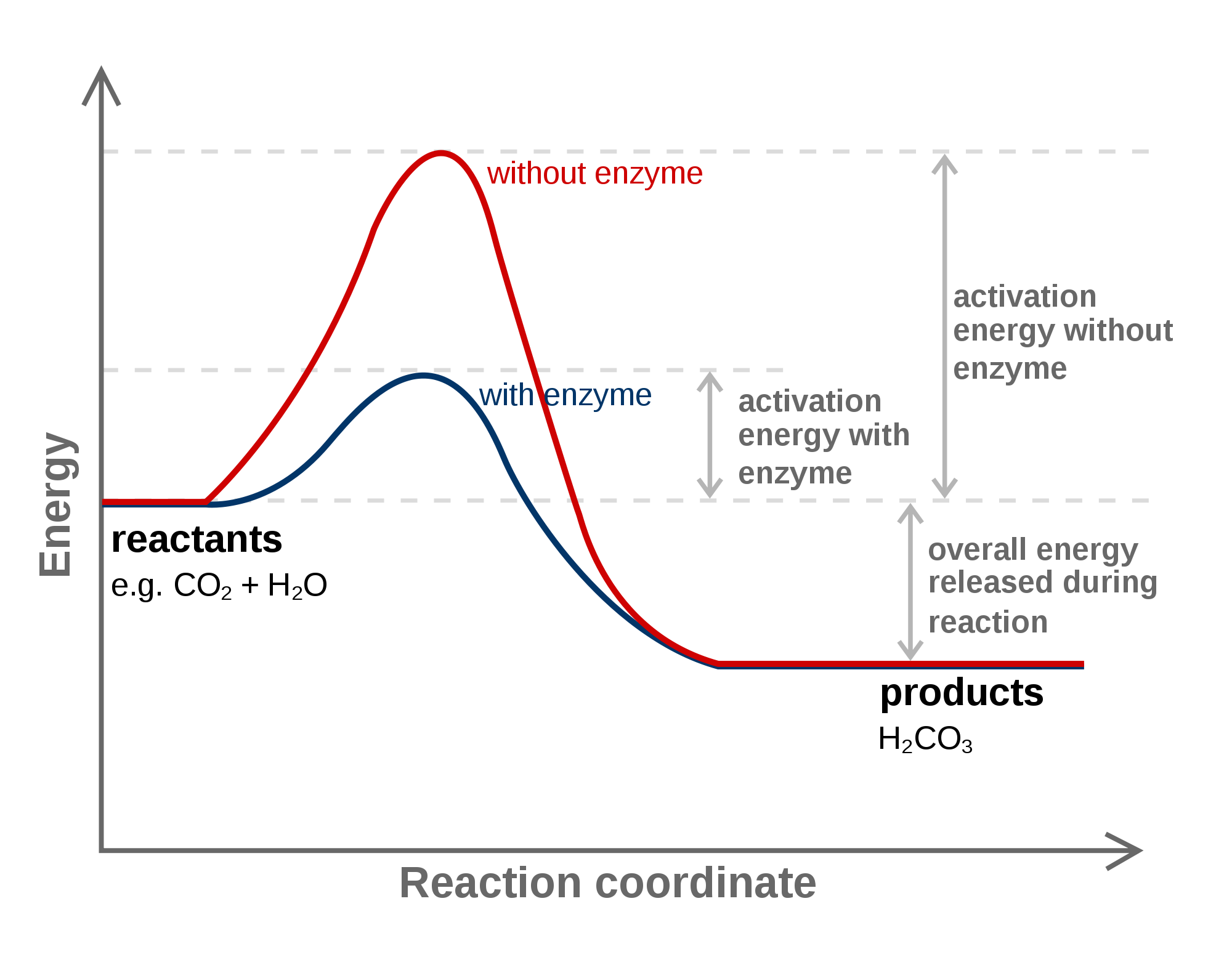
Catalyst To A Chemical Reaction Provides An Alternate Pathway That . This does not change the frequency of collisions. Catalysts are conventionally divided into two categories: so catalysts provide an alternative pathway by allowing a new intermediate to be formed. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. a catalyst provides an alternative. the catalyst provides a different reaction path with a lower activation energy. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. catalysts provide alternative reaction pathways.
from circuitdbplastered.z13.web.core.windows.net
Catalysts are conventionally divided into two categories: so catalysts provide an alternative pathway by allowing a new intermediate to be formed. catalysts provide alternative reaction pathways. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. a catalyst provides an alternative. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. This does not change the frequency of collisions. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. the catalyst provides a different reaction path with a lower activation energy.
Reaction Energy Diagram With Catalyst
Catalyst To A Chemical Reaction Provides An Alternate Pathway That this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. This does not change the frequency of collisions. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. catalysts provide alternative reaction pathways. Catalysts are conventionally divided into two categories: a catalyst provides an alternative. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. the catalyst provides a different reaction path with a lower activation energy.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst To A Chemical Reaction Provides An Alternate Pathway That This does not change the frequency of collisions. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. catalysts provide alternative reaction pathways. Catalysts are conventionally divided into two categories: catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. this difference illustrates. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalyst To A Chemical Reaction Provides An Alternate Pathway That catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the.. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From ceqbzvop.blob.core.windows.net
Catalysts Of Chemical Reactions at Kimberly Ray blog Catalyst To A Chemical Reaction Provides An Alternate Pathway That the catalyst provides a different reaction path with a lower activation energy. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. a catalyst provides an alternative. Catalysts. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From circuitdiagramlows.z22.web.core.windows.net
Catalyst Energy Diagram Catalyst To A Chemical Reaction Provides An Alternate Pathway That catalysts provide alternative reaction pathways. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. Catalysts are conventionally divided into two categories: a catalyst provides an alternative. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts function by providing an. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From slideplayer.com
Reaction Mechanism The reaction mechanism is the series of elementary Catalyst To A Chemical Reaction Provides An Alternate Pathway That catalysts provide alternative reaction pathways. Catalysts are conventionally divided into two categories: a catalyst provides an alternative. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. . Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Catalyst To A Chemical Reaction Provides An Alternate Pathway That catalysts provide alternative reaction pathways. the catalyst provides a different reaction path with a lower activation energy. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst To A Chemical Reaction Provides An Alternate Pathway That this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. the catalyst provides a different reaction path with a lower activation energy. This does not change the frequency of collisions. Catalysts are conventionally divided into two categories: catalysts function by providing an alternate reaction mechanism that has a. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst To A Chemical Reaction Provides An Alternate Pathway That catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts provide alternative reaction pathways. This does not change the frequency of collisions. the catalyst provides a different reaction path with a lower activation energy. this difference illustrates the means by which a catalyst functions to accelerate. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst To A Chemical Reaction Provides An Alternate Pathway That this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. Catalysts are conventionally divided into two categories: This does not change the frequency of collisions.. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalyst To A Chemical Reaction Provides An Alternate Pathway That so catalysts provide an alternative pathway by allowing a new intermediate to be formed. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. This does not change the frequency of collisions. catalysts provide alternative reaction pathways. catalysts function by providing an alternate reaction mechanism that has. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Catalyst To A Chemical Reaction Provides An Alternate Pathway That a catalyst provides an alternative. catalysts provide alternative reaction pathways. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. the catalyst provides a different reaction path with a lower activation energy.. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst To A Chemical Reaction Provides An Alternate Pathway That catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. the catalyst provides a different reaction path with a lower activation energy. this difference illustrates the means by which a catalyst functions to. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst To A Chemical Reaction Provides An Alternate Pathway That a catalyst provides an alternative. This does not change the frequency of collisions. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts provide alternative reaction pathways. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst To A Chemical Reaction Provides An Alternate Pathway That a catalyst provides an alternative. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. catalysts provide alternative reaction pathways. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. catalysts increase the rate of a reaction by lowering the activation energy. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst To A Chemical Reaction Provides An Alternate Pathway That This does not change the frequency of collisions. a catalyst provides an alternative. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. so catalysts provide an alternative pathway by allowing a new intermediate to be formed. the catalyst provides a different reaction path with a lower. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst To A Chemical Reaction Provides An Alternate Pathway That Catalysts are conventionally divided into two categories: this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction. a catalyst provides an alternative. the catalyst provides a different reaction path with a lower activation energy. so catalysts provide an alternative pathway by allowing a new intermediate to be. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst To A Chemical Reaction Provides An Alternate Pathway That the catalyst provides a different reaction path with a lower activation energy. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be found in the absence of the catalyst. Catalysts are conventionally divided into two categories: so catalysts provide an alternative pathway by allowing a new intermediate to be formed.. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.
From www.slideshare.net
Biology 2.4 Catalyst To A Chemical Reaction Provides An Alternate Pathway That Catalysts are conventionally divided into two categories: so catalysts provide an alternative pathway by allowing a new intermediate to be formed. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts function by providing an alternate reaction mechanism that has a lower activation energy than would be. Catalyst To A Chemical Reaction Provides An Alternate Pathway That.